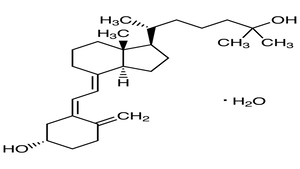
On February 21, Regulation (EU) 2025/352 was published, which modifies Directive 2002/46/EC of the European Parliament and of the Council of June 10, 2002, on the approximation of the laws of the Member States relating to food supplements with regard to the inclusion of calcidiol monohydrate as a new source of vitamin D permitted in the manufacture of food supplements.
Calcidiol monohydrate was already authorized as a novel food on April 10, 2024 by Commission Implementing Regulation (EU) 2024/1052 and under the following conditions:
-
- The designation of the novel food on the labelling of food products containing it will be calcidiol (calcifediol) monohydrate (vitamin D).
-
- Specific food category in which it can be used: food supplements, as defined in Directive 2002/46/EC, except those intended for infants and young children.
-
- Maximum levels:
-
-
- 10 μg/day for children from 11 years of age and adults
-
-
-
- 5 μg/day for children from 3 to 10 years of age
-
-
- Only DSM Nutritional Products Ltd. will be authorised to place the novel food on the market in the Union for a period of five years from 1 May 2024, unless a subsequent applicant obtains an authorisation for that novel food without referring to the protected scientific data or has referred to them with the prior consent of DSM Nutritional Products Ltd.